As part of its organizational restructuring effort to protect the health of the consumers, the Ministry of Health and Welfare (formerly known as the Department of Health, Executive Yuan) combined the organizations formerly known as the Bureau of Food Safey, Bureau of Pharmaceutical Affairs, Bureau of Drug and Food Analysis and Bureau of Controlled Drugs under the Department of Health into the Food and Drug Administration, Department of Health on January 01 of 2010. On July 23 of 2013, as part of the Executive Yuan’s organizational restructuring, the Taiwan Food and Drug Administration under the Ministry of Health and Welfare was established. The restructured organization not only greatly shortened the time taken to plan and implement management policies, but also promoted the transparent, rapid and diverse disclosure of food and drug information, guaranteeing safety of the quality of food, drug, medical device and cosmetic products for all consumers.
Service items
Pharmaceutical Management
The pharmaceutical management framework encompasses procedures from product development to market approval, including R&D, pre-clinical studies, clinical trials, application for market approval, manufacturing and post-market management. Each of these steps shall comply with various good practice (GxP) standards and regulations. In addition, comprehensive pharmaceutical management policies has been established through the harmonization of international regulations, production sources control, pre-marketing control, post-marketing surveillance, and management of product distribution and pharmaceutical vendors. These are all effective strategies to ensure the safety, efficacy and quality of the pharmaceutical products.
Medical Devices Management
The framework of medical device management is centered on protection of consumers. It establishes corresponding regulatory mechanisms for the phases from product design, pre-clinical testing, clinical trial, pre-market application, to marketing. By covering fully the “design,” “production,” and “sale” of total product life cycle management system, the safety, effectiveness, and quality of medical devices are efficiently managed. Furthermore, to respond to the international regulatory trends and cope with the ever-changing environment, regulatory policies that may keep pace with the times and can be in line with international trends are being developed at an accelerated rate to promote the industry development.
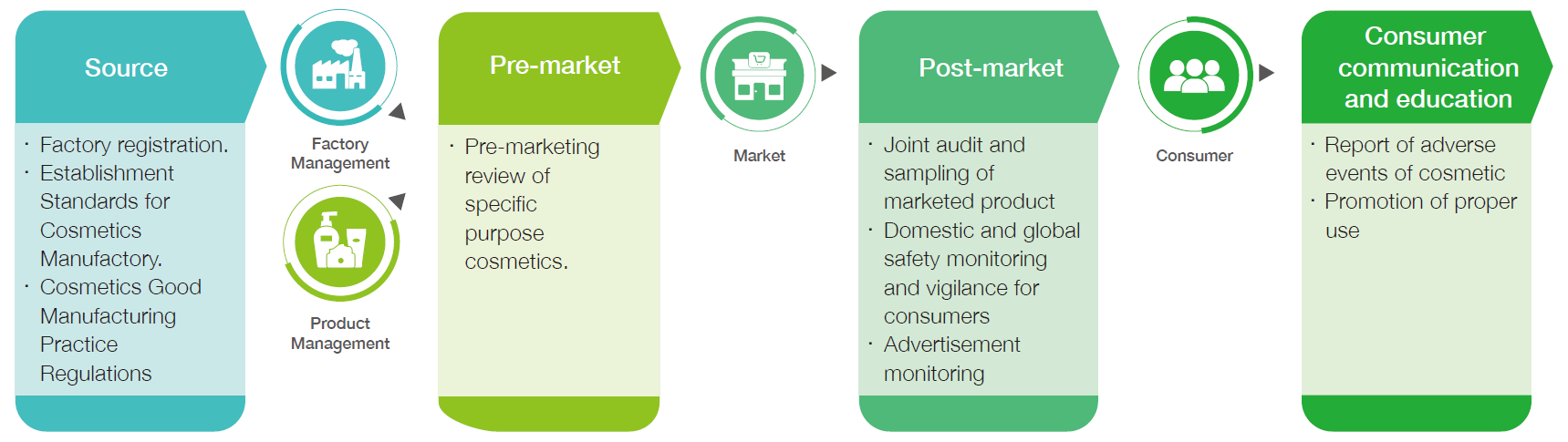
Cosmetic Management
The cosmetic management system is divided into production source control, pre-market management, and post-market surveillance. Source control management includes ensuring that manufactories comply with the Establishment Standards for Cosmetics Manufactory and Cosmetics Good Manufacturing Practice Regulations (GMP). Pre-market management includes registration specific-purpose cosmetics. Post-market surveillance that focuses on implementing cosmetics quality surveillance programs, joint audits spanning multiple counties and cities, establishing a product adverse event reporting system for cosmetics, monitoring of domestic and global cosmetic safety alerts regularly, and strengthening consumer awareness of safe cosmetics use to create a comprehensive cosmetics quality and safety protection network.